Japanese researchers at Yokohama National University have presented a promising alternative to nickel and cobalt-based batteries for electric vehicles (EVs).
Their approach uses manganese in the anode to create a high energy density battery that is both cost-effective and sustainable.
Electric vehicle manufacturers prefer nickel and cobalt batteries because they offer higher energy density, meaning longer range from a smaller battery pack. However, both components are expensive to source and relatively rare, making them an unsustainable option as electric vehicle use increases rapidly around the world.
Lithium-ion (Li-ion) batteries are the preferred battery option for most electronic devices. However, their lower energy density puts them at a disadvantage in electric vehicles. Research and development efforts to improve them have led to the introduction of better Li-ion options.
Experiments have also been carried out using manganese as an anode material alongside lithium, such as LiMnO2. However, applications have been limited due to the inferior performance of the electrode. Researchers at Yokohama National University (YNU) in Japan have addressed this issue in their recent work.
Working with a monoclinic system
After extensively studying LiMnO2 in its various forms using X-ray diffraction, scanning electron microscopy and electrochemical methods, researcher Naoaki Yabuuchi and his team at YNU found that a monoclinic layered domain activates the structural transition of LiMnO2 into a spinel-like phase. A monoclinic system is a type of group symmetry of a solid crystalline structure.
LiMnO2 improves the performance of the electrode material by facilitating the phase transition. Without the phase transition, a LiMnO2 electrode will have suboptimal performance.
“Based on these findings, nanostructured LiMnO2 with monoclinic layered domain structures and high surface area could be directly synthesized using a simple solid-state reaction,” Yabuuchi said in a press release.
The reaction has no intermediate steps and can be synthesized directly from two components using a calcination process.
Performance improvements with Mn
Post-synthesis testing showed that a battery with a LiMnO2 electrode achieved an energy density of 820 watt-hours per kilogram (Wh kg-1), compared to 750 Wh per kg for a nickel-based battery. Only lithium-based batteries have an even lower energy density of 500 Wh per kg.
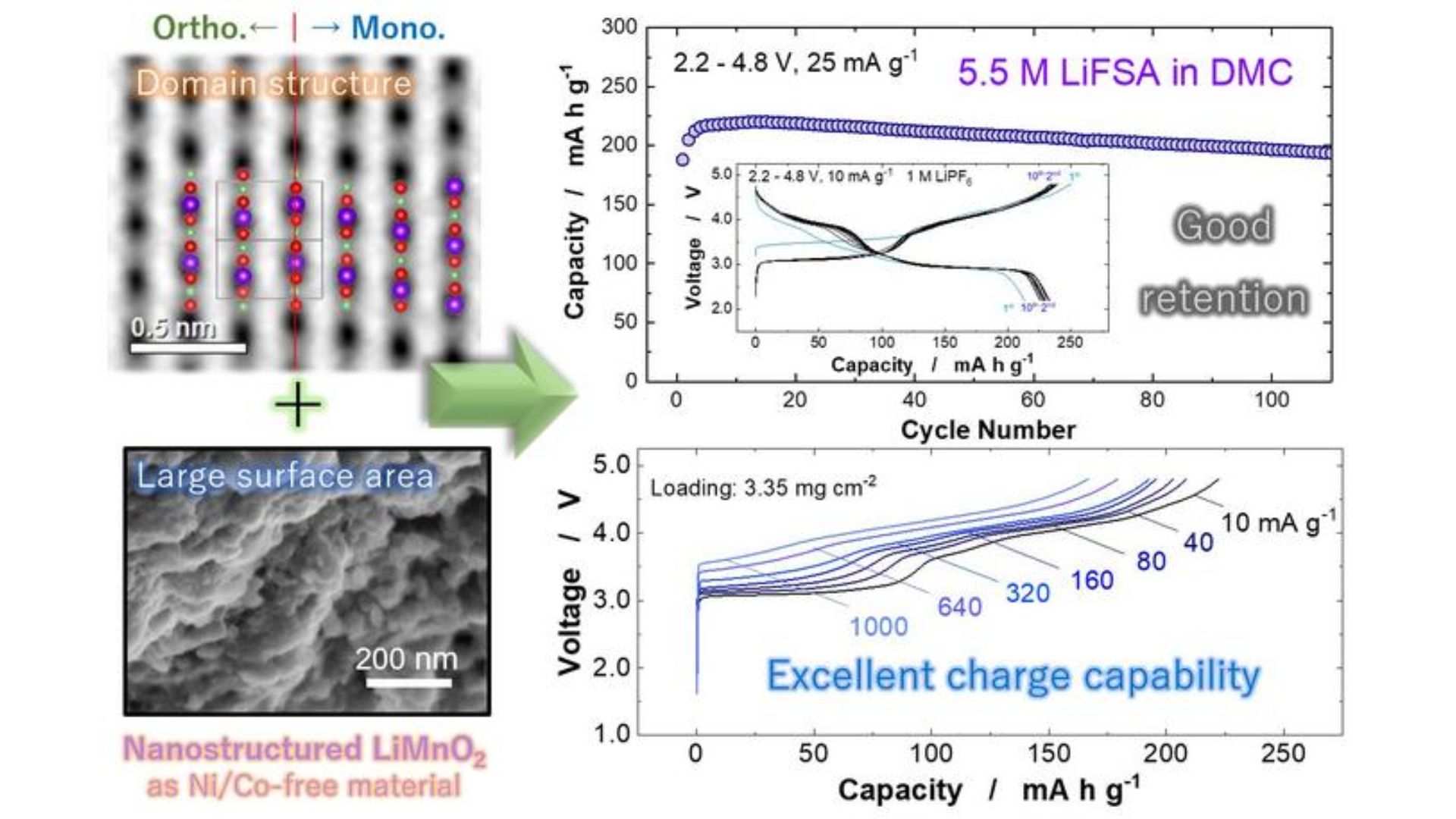
The researchers told Interesting technology in an email that manganese, when used in other polymorphs, typically has only half the energy density capacity.
Previous work with manganese reported a voltage drop in batteries, where the voltage output decreased over time, reducing the performance of the electronic device. However, the researchers did not observe such results with the LiMnO2 electrode.
Dissolution of manganese can still occur, either through phase changes or a reaction with an acidic solution. The press release goes on to say that the researchers plan to address this problem using a highly concentrated electrolyte solution and a lithium phosphate coating.
The researchers believe their work has contributed to the development of a new offering that is competitive with existing options, sustainable in production and environmentally friendly in the long term. They look forward to commercializing their technology and deploying it in the electric vehicle industry.
The research team added in their email to IE: “We found a very cost-effective methodology and this is the important result of our study.”
ABOUT THE PUBLISHER
Ameya Paleja Ameya is a science writer based in Hyderabad, India. He is a molecular biologist at heart, but during the pandemic he traded the micropipette for writing about science and doesn’t want to go back. He enjoys writing about genetics, microbes, technology and public policy.