Researchers have developed a sustainable manganese-based lithium-ion battery that could revolutionize the electric vehicle industry.
Published in ACS Central ScienceThe study highlights a breakthrough in the use of nanostructured LiMnO2 with monoclinic symmetry to improve battery performance and stability without the typical voltage drop. This innovation not only improves fast charging capability but also addresses the long-standing problem of manganese dissolution.
Manganese-based lithium-ion batteries
Lithium-ion batteries (or Li-ion batteries) are heavyweights in the world of rechargeable batteries. As electric vehicles become more common around the world, a high-energy, low-cost battery that takes advantage of abundant manganese (Mn) can be a sustainable option that can be commercially available and used in the automotive industry.
Currently, the batteries used to power electric vehicles are based on nickel (Ni) and cobalt (Co), which can be expensive and unsustainable for a society with a growing desire for electric vehicles. By switching the positive electrode materials to lithium/manganese-based material, researchers hope to retain the high performance of Ni/Co-based materials, but with a low-cost, sustainable option.
The researchers publish their results today (26 August) in ACS Central Science.
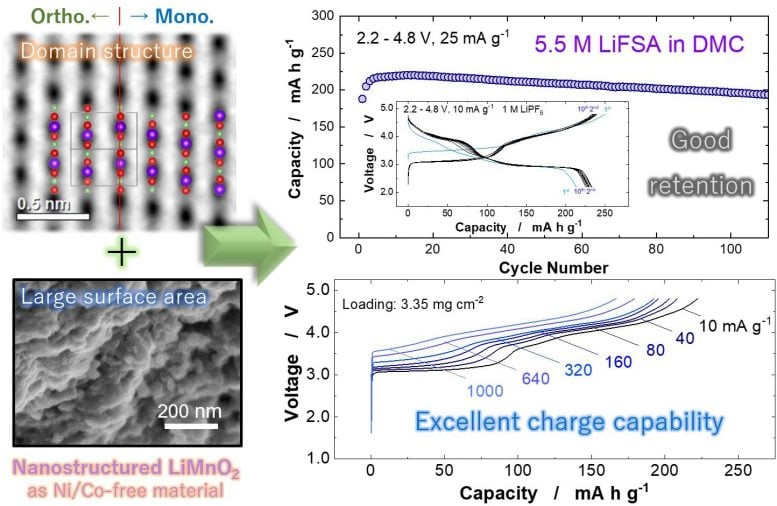
Innovations in electrode materials
Lithium-ion batteries are nothing new in the field of rechargeable electronics, but there are always ways to innovate and improve already reliable methods. LiMnO2 as an electrode material has been investigated in the past, but was always limited by the restricted electrode performance.
“Through the systematic investigation of various LiMnO2 Polymorphs showed that the monoclinic layered domain effectively activates the structural transition to the spinel-like phase. Based on this finding, nanostructured LiMnO2 with the monoclinic, layered domain structures and high surface area was directly synthesized by a simple solid-state reaction,” said Naoaki Yabuuchi, author and researcher of the study.
Breakthrough in monoclinic LiMnO2 structures
A monoclinic system refers to the type of group symmetry of a solid crystalline structure. A Li/Mn arrangement with the monoclinic symmetry seems to be the key to the preparation of LiMnO.2 a viable option for a positive electrode material. Without the structural phase transition enabled by the monoclinic domain, the electrode performance would be limited due to the suboptimal crystal structure of LiMnO2 and accompanying phase transitions.
Improving the performance of electric vehicle batteries
After observing and testing the different polymorphs, it was found that the required structure can be synthesized directly from two components without the need for an intermediate step. The resulting material is competitive with nickel-based layered materials and has excellent fast-charging capabilities, which are essential for electric vehicles.
The nanostructured LiMnO2 with the monoclinic layered domain is synthesized by a simple calcination process to obtain a high energy density product reaching 820 watt hours per kilogram (Wh kg).-1), compared to about 750 Wh kg-1 for nickel-based coating materials and 500 Wh kg-1 for other low-cost lithium-based materials.
Stability and durability of nanostructured LiMnO2
There is also no reported voltage drop when using nanostructured LiMnO2which is common in manganese-based materials. Voltage droop is a phenomenon in which voltage gradually decreases, which reduces the performance and responsiveness of an electronic component over time. However, with nanostructured LiMnO, this does not seem to be a noticeable problem.2which is the subject of the study.
Addressing practical challenges
Although there are promising results, there is a practical problem to be observed: the dissolution of manganese. Over time, manganese can dissolve due to many factors, such as phase changes or reactions with acidic solutions. Fortunately, this can be contained or completely mitigated by using a highly concentrated electrolyte solution and a lithium phosphate coating.
The researchers hope that their findings will contribute to a more sustainable energy source than fossil fuels, especially with regard to electric vehicles. The performance of LiMnO2with its competitive energy density compared to nickel-based materials, shows the potential of alternative materials to produce environmentally friendly products that are sustainable both in production and as a long-term investment. An ideal future for nanostructured LiMnO2-based electrode materials would enable commercialization and industrial production in the luxury electric vehicle industry.
Reference: “A practical and sustainable Ni/Co-free high-energy electrode material: Nanostructured LiMnO2” August 26, 2024, ACS Central Science.
DOI: 10.1021/acscentsci.4c00578
Yuka Miyaoka, Yuna Oguro, Yosuke Ugata and Naoaki Yabuuchi from the Department of Chemistry and Life Science at Yokohama National University with Yosuke Ugata and Naoaki Yabuuchi also from the Advanced Chemical Energy Research Center at Yokohama National University, Takahito Sato from the Department of Applied Chemistry at Tokyo Denki University, Sayaka Kondo, Koki Nakano and Masanobu Nakayama from the Frontier Research Institute for Materials Science at Nagoya Institute of Technology, Damian Goonetilleke and Neeraj Sharma from the School of Chemistry at the University of New South Wales, Alexey M. Glushenkov from the Research School of Chemistry at Australian National UniversitySatoshi Hiroi and Koji Ohara of the Department of Energy Materials at Shimane University, Koji Takada and Yasuhiro Fujii of Tosoh Corporation contributed to this research.
JSPS, Grant for Scientific Research, JST, MEXT Program: Materials Research and Development Data Creation and Utilization Project, JST, Establishing Sustainable Partnerships for an Innovative Research Ecosystem (ASPIRE), JST, Green Technologies for Excellence (GteX) Program, Australian Research Council, Photon Factory Program Advisory Committee, Japan Synchrotron Radiation Research Institute (JASRI), Aichi Synchrotron Radiation Center, and Australian Nuclear Science and Technology Organisation (ANSTO) made this research possible.